In accordance with the thematic education deployment requirements of the Party Central Committee and the Provincial Party Committee and the work arrangements of the Municipal Party Committee, today (November 23), You Jianfeng, member of the Standing Committee of the District Party Committee, Director of the Propaganda Department, and Director of the United Front Work Department, launched the "Thousands of Villages, Thousands of Enterprises, Thousands of Homes" campaign in Yuexi Subdistrict. Visit every household, go deep into the grassroots, into enterprises, and into the masses, strengthen services, promote development, observe people's conditions, and resolve people's concerns. He emphasized that we must adhere to the guidance of Xi Jinping Thought on Socialism with Chinese Characteristics for a New Era, proactively connect with enterprises, ask for their needs and serve them, and use practical measures to help enterprises solve practical difficulties and continuously enhance their sense of gain and satisfaction.
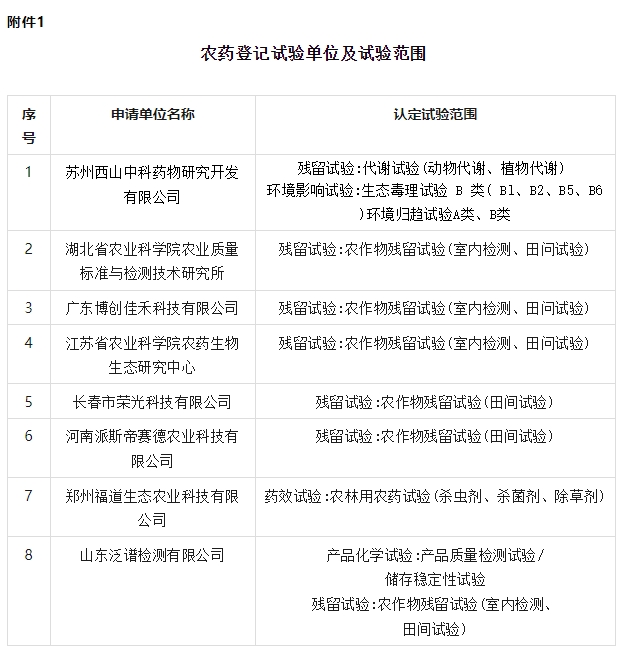
Suzhou Xishan Zhongke Pharmaceutical Research and Development Co., Ltd.
The company is committed to providing global customers with a full range of high-tech research services on safety evaluation, ecotoxicology testing, pharmacodynamics and pharmacokinetics of drugs, pesticides and chemicals. Organization for GLP testing of chemicals. It undertakes the research tasks of the Suzhou Biomedical Service Platform Construction Project and the Suzhou Science and Technology Plan Key Industrial Technology Innovation Project. It is a high-tech enterprise in Jiangsu Province, a R&D enterprise in Jiangsu Province, and the Jiangsu Province Pesticide Toxicology Evaluation and Risk Assessment Engineering Technology Research Center.

You Jianfeng walked into the laboratory and workshop, communicated cordially with scientific researchers and staff, and gained an in-depth understanding of the company's main projects and specific research and development processes. Focusing on the development prospects of the company, You Jianfeng said that the company must strengthen confidence, be determined to innovate, further strengthen research in areas of expertise, continue to promote technological progress, and promote the company to become stronger and better with higher product quality and better services. .